Chemistry of Plastics
A Short Chemistry Lesson
The idea of plastic came from naturally accruing substances known as polymer. Polymers can be found existing in nature in the form of tar, shellac, tortoise shells, horns, tree saps such as amber and latex.
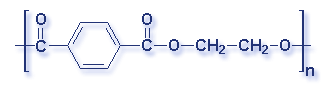
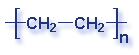
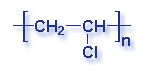
Though what is a polymer? A substance made of many repeating chemical units or molecules. A simpler way to think of polymers is as a paper clip chain. The paper clips are all made out of the same things, as they are linked together it illustrates the concept of polymers chemical unit repeating themselves. Depending on the substances used depends on the polymer created. Most polymers contain carbon and hydrogen, though they can also have oxygen, chlorine, fluorine, nitrogen, silicon, phosphorous and sulfur. PVC contains chlorine, Nylon contains nitrogen, Teflon contains fluorine, and polyester and polycarbonates contain oxygen
Polymers each have distinct traits but most polymers tend to have the same characteristic. Polymers tend to be resistant to chemicals, can be thermal and electrical insulators, for the most part light weight with varying degrees of strength, as well they are able to be made into a heavy walled jar or a thin fiber.
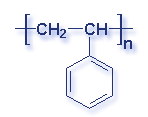
Molecular Structure of Common Plastics

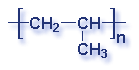
Chat with Us






 Shop All Packaging
Shop All Packaging Glass Containers
Glass Containers Plastic Containers
Plastic Containers Metal Containers
Metal Containers Cardboard Containers
Cardboard Containers Industrial Containers
Industrial Containers Caps / Closures
Caps / Closures Shrink Bands
Shrink Bands Design Custom Labels
Design Custom Labels Subscribe & Save Orders
Subscribe & Save Orders Shop By Industry
Shop By Industry Custom Packaging
Custom Packaging Pallet Qty Packaging
Pallet Qty Packaging SKS Direct Wholesale
SKS Direct Wholesale Packaging Equipment
Packaging Equipment Help/Info
Help/Info New Products
New Products Promotions
Promotions Newsletters
Newsletters Combo Kit Deals
Combo Kit Deals Product Closeouts
Product Closeouts Recently Back In Stock
Recently Back In Stock










